Join our pilot program!
We have launched a pilot program for Phagenomics BatchQC! Joining the program allows you to test BatchQC in a non-GxP environment and gives you the opportunity to affect development prior to official release. We'd be thrilled to have you on board!
Main Capabilities
Genomic traceability
Monitor genomic integrity from batch to batch with automated mutation and contamination detection workflows. Compatible with Illumina, PacBio and Nanopore technologies.
Compliance built-in
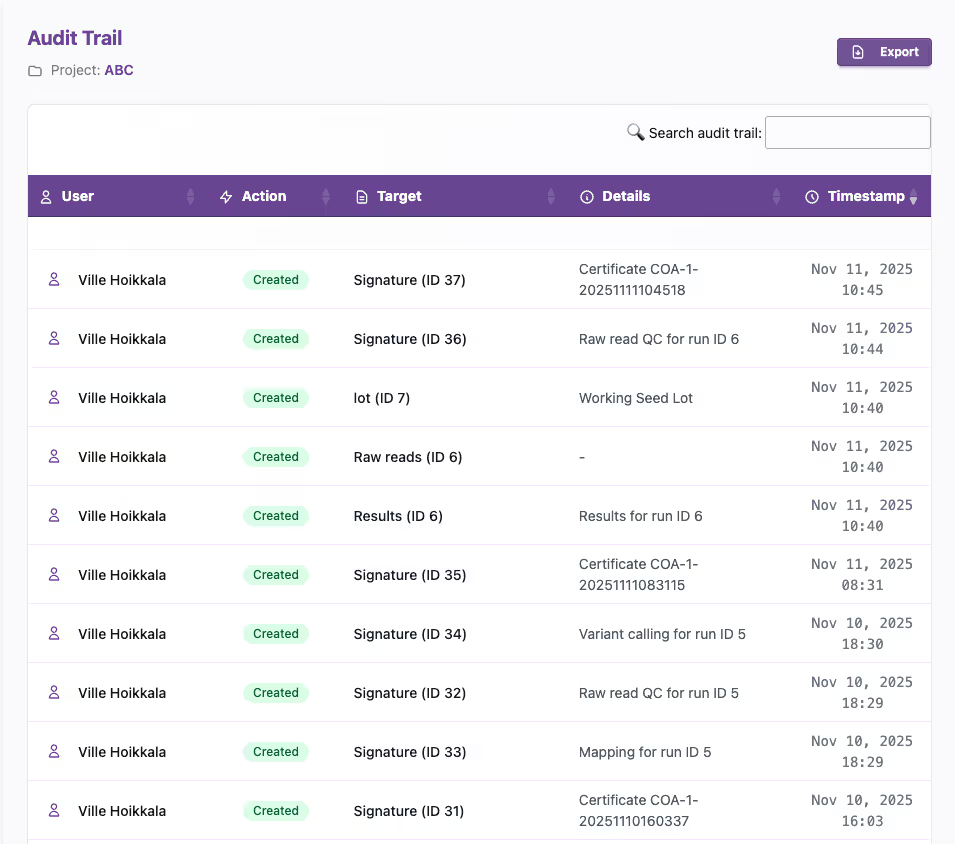
BatchQC reduces uncertainty in your compliance efforts through FDA CFR 21 part 11 compliant digital signatures, audit trails and exportable Certificates of Analysis.
Customisable to your workflow
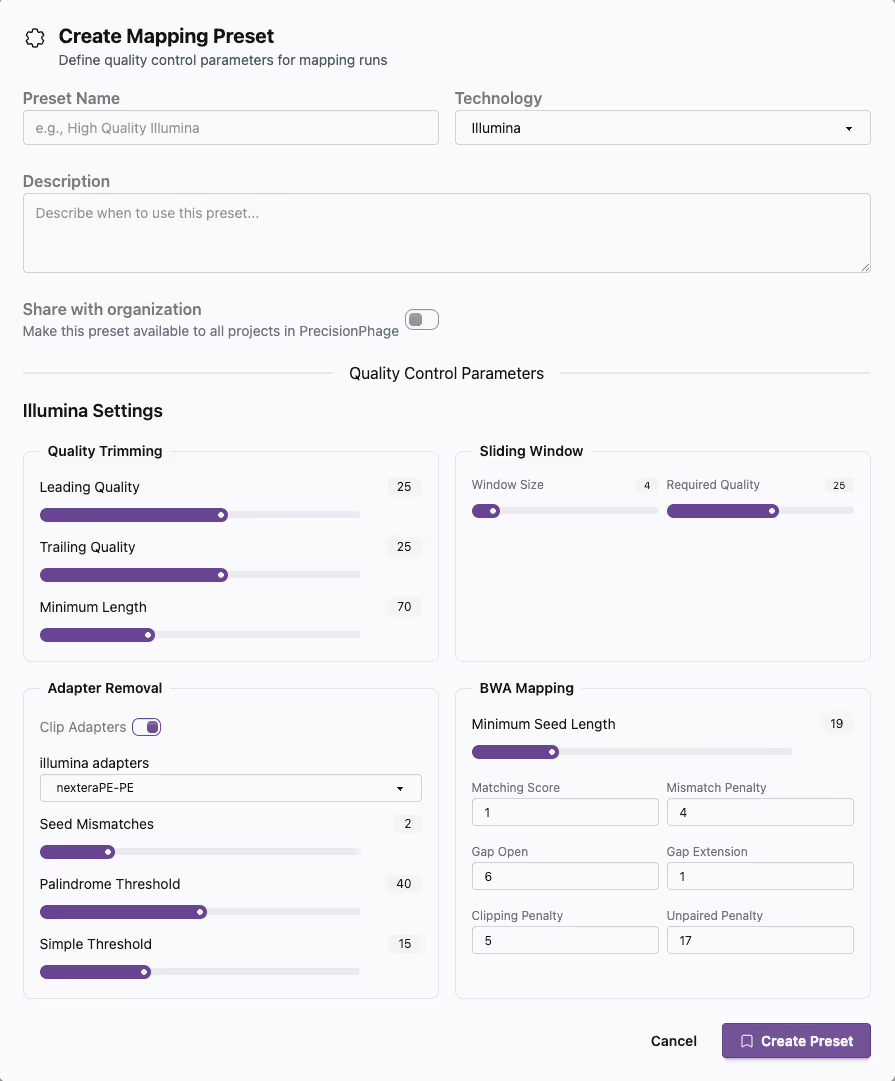
Customisable lot architectures, metadata, quality control rules and company policies. BatchQC sits in your production workflow and simplifies your compliance efforts.
Supported by experts
From validation to training, we are making BatchQC the standard for GMP-ready phage production. The software is built completely in-house by a dedicated team of phage scientists, software engineers and quality specialists.
Full Traceability Across
the Production Chain
BatchQC verifies genetic consistency from Master Seed Lot to final production batch. Automated checks ensure lot-to-lot uniformity and rapid deviation detection.
Master Seed
The starting point of your phage production, containing a pre-annotated phage genome as a GenBank file.
Working Seed
Following the expected upcoming regulatory guidelines, subsequent lots are sequenced and compared to the Master Seed Lot using our automated pipelines.
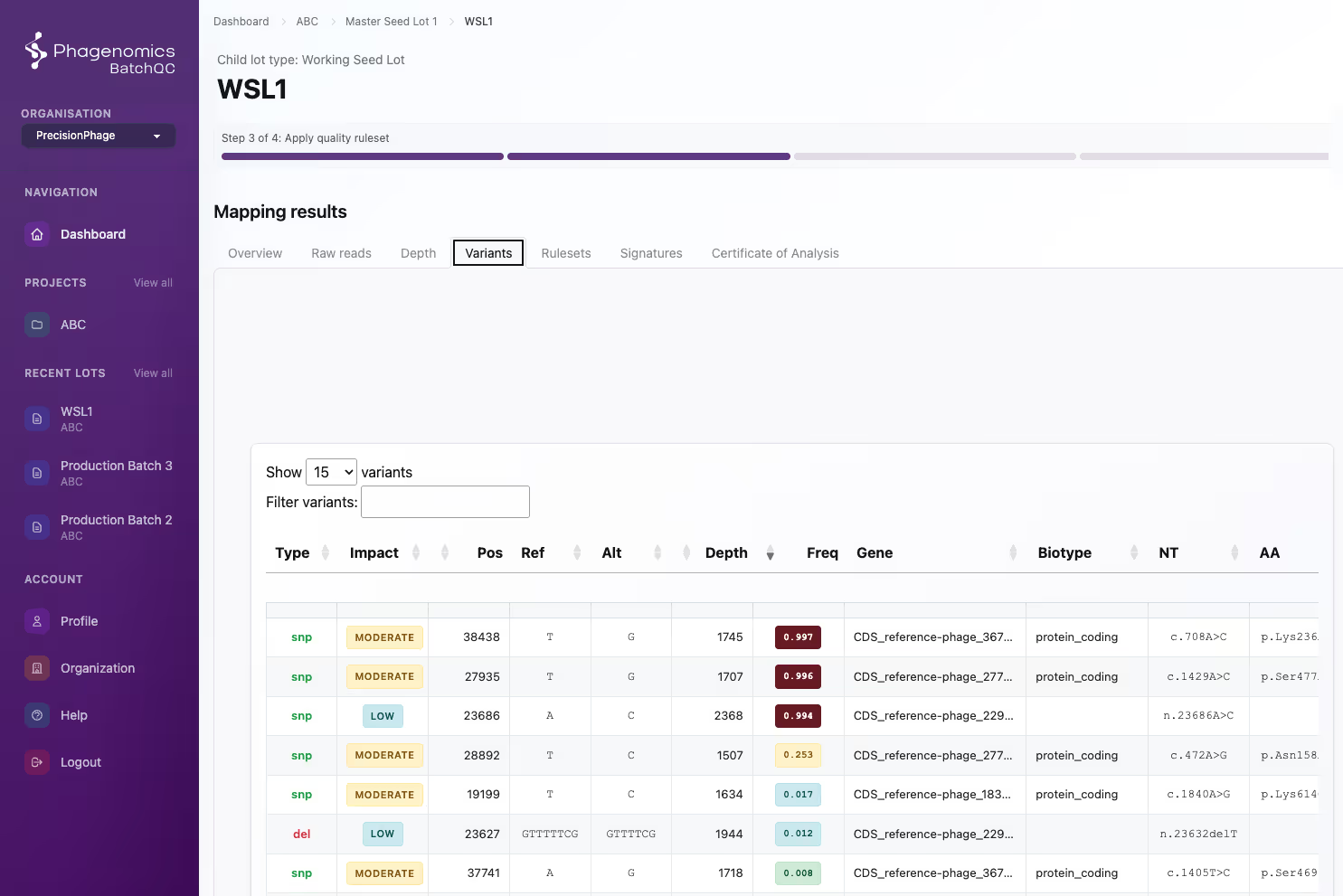
Results
+
CoA
Production Batch
The Production Batch is similarly sequenced and compared to the Master Seed Lot. A Certificate of Analysis is produced, containing results with digital signatures.
Results
+
CoA
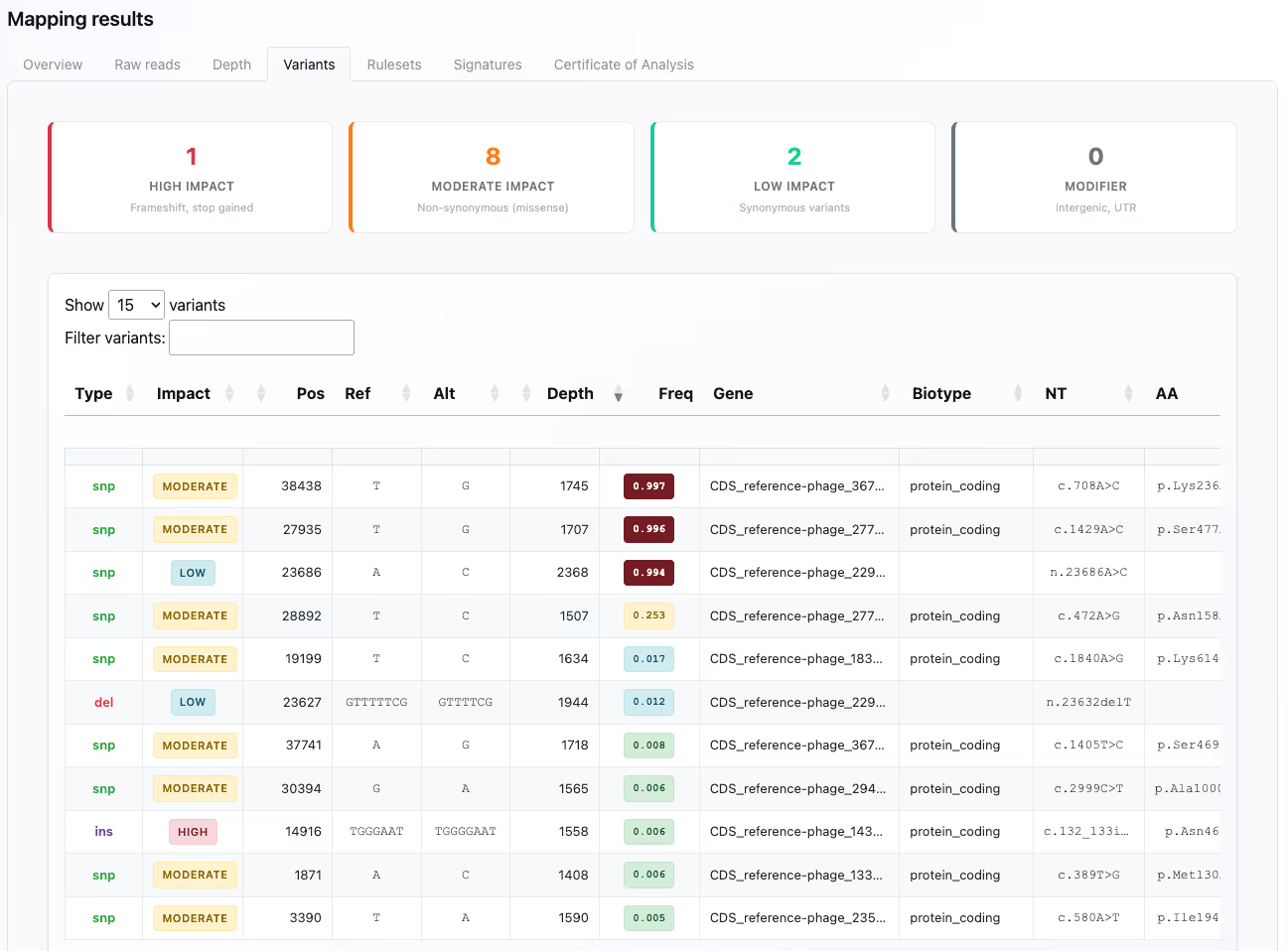
Regulatory-Ready Certificates of Analysis
BatchQC transforms raw QC results into validated Certificates of Analysis that meet GMP and 21 CFR Part 11 requirements. Each report consolidates genome verification, contamination checks, and batch metadata within a single, traceable document.



Enterprise-Grade Security Inside the EU
Phagenomics BatchQC runs on state-of-the-art EU-based infrastructure provided by ISO27001 certified partners. Data safety is guaranteed by encryption at rest and at transit.
We are known for...
as the first principle.
Easy-to-use software that your quality team can run — no IT involvement required.
Don’t let genomic traceability become your bottleneck.
We’ll be with you through training, validation and maintenance.
Phagenomics BatchQC Pricing
Flexible plans for people pushing the boundaries of genomic science. Be one of the first to take advantage of BatchQC. Apply to our Pilot program.

Lead the way
FAQs
If your question is not listed below, don’t hesitate to contact us!
Yes, we offer service-level agreements (SLAs). In line with GAMP 5 (2nd edition) principles, our SLAs help demonstrate control, reliability, and compliance by clearly defining system availability, support response times, and service continuity.
BatchQC is a cloud-based SaaS solution, which means it can be accessed from any device with an internet connection and a modern web browser. This eliminates the need for specific hardware or software installations, making it easy to deploy and use across your organization.
Yes! From the outset, BatchQC was designed with flexibility in mind. You can customize various aspects of the system, including the number and naming of tiered production levels (such as Master Seed Lot and Working Seed Lot), organizational policies related to user roles and privileges. You also have the ability to tailor the details of quality control gates down to the tiniest thresholds (or use our reasonable default values) and add custom metadata fields to your batches, according to your traceability needs.
We have a defined Software Configuration Management and Change control procedures for performing updates to ensure that the system continues to operate in compliance with its validated state, with re-validation performed when necessary. Minor updates, such as security patches or small bug fixes that are non-GxP-relevant, are applied regularly, with change logs made available to users. For updates that may have GxP-relevant effects, we provide change notifications several months in advance. Additionally, major updates are accompanied by access to a separate test environment, allowing you to test and validate the enhanced functionality before it is applied to the GxP production environment. This approach helps ensure operational continuity and supports your compliance efforts.
We provide comprehensive support and training to ensure a smooth implementation and ongoing use of BatchQC. This includes detailed documentation, online training sessions, and access to a dedicated support team to assist with any questions or issues. We also offer regular updates and enhancements to keep your system up-to-date with the latest features and improvements.
At present, managing genomic integrity at the GMP level for phage production often relies on generalized bioinformatic tools and regulatory processes that are dispersed across various departments. Developing a validated process from scratch using these non-specialized tools can be time-consuming, costly, and fraught with inherent risks. BatchQC addresses these challenges as the first quality control management system specifically designed for GMP-level phage production, streamlining processes and reducing associated risks by combining batch management, bioinformatic workflows and documentation governance into one centralised tool.
We are soon launching a pilot program that provides access to non-GxP beta versions of Phagenomics BatchQC. By joining now, you can test the software's adaptability to your workflows and have the opportunity to influence features before the official launch.
Yes, Phagenomics BatchQC is designed in accordance with the Good Automated Manufacturing Practice (GAMP 5, 2nd edition) guidelines, with a strong focus on audits and validation. As part of our service, we offer comprehensive validation materials to assist you in the validation process with your local authorities.
Let’s Talk!
Let’s start your compliance journey together! By contacting us, you will be directly connected with the developers, project managers, and quality managers of BatchQC. Pilot program queries can be sent through the same form.

Hoikkala

Bruneaux

Latikka